The development and introduction of new biological drug substances has been on the rise, and so have the regulatory requirements placed on these often life-saving products. At the same time, innovative analytical technologies have been developed that offer new analytical capabilities.
As the percentage of large proteins (like monoclonal antibodies or high molecular weight polypeptides) continues to increase, a particular challenge is becoming increasingly evident in the development and supervision of processes: particle analysis.
For various reasons, particles can form in injectable drugs during the manufacturing process or transportation. Biologics are often particularly prone to particle formation. The reason for that can be the inherent instability of the drug substance in the actual formulation, or the manufacturing process itself, for instance by the emergence of strong shear forces. Both could result in a denatured protein.
Denatured proteins can be inactive, in which case the drug may become ineffective. Furthermore, visible or sub-visible particles can produce unwanted immunological reactions in patients, which might trigger the drug’s side-effects.
Regulatory authorities have responded to these facts by reinforcing particle-related regulations. In the USA, for instance, the corresponding regulations listed in chapters <790> and <1790> of the United States Pharmacopeia (USP) have been reviewed and made stricter. The authorities now offer some orientation when it comes to the visual inspection of filled units with regards to visible particles in injectable drugs.
Corresponding regulations have been introduced by the European Medicines Agency (EMA). In addition, the sections of the corresponding pharmacopoeias covering sub-visible particles are currently being reviewed and expanded where needed.
Particle characterization

Today, regulatory authorities expect manufacturing and transportation processes to be developed, validated and set up in such a way as to minimize particle formation or other contaminations. To guarantee this demands not only profound knowledge of the processes is crucial, but also scientific expertise in characterizing and identifying particles. Meshing this knowledge allows developing reliable manufacturing processes, ready for filing.
In addition to protein denaturing, there are other causes of particle formation, such as the introduction of cellulose fibers during cleaning processes, friction with metal or Teflon, materials such as silicone oil or rubber, which are part of the primary packaging systems. The particle sizes extend from sub-visible (nanometer to the low micrometer range) to visible (about 100 - 150 µm).
The current standard technique to determine sub-visible particles is the measurement using a Hiac Royco. This instrument can count the number and size of sub-visible particles in the 1-100 µm range. Characterizing or identifying the particles is not possible. The standard procedure to determine visible particles is a manual visual control of the filled units and the subjective description of whatever visible contamination shows up.
Different analytical technologies have advanced the science on particle analysis and can deliver extensive particle characterization and identification:
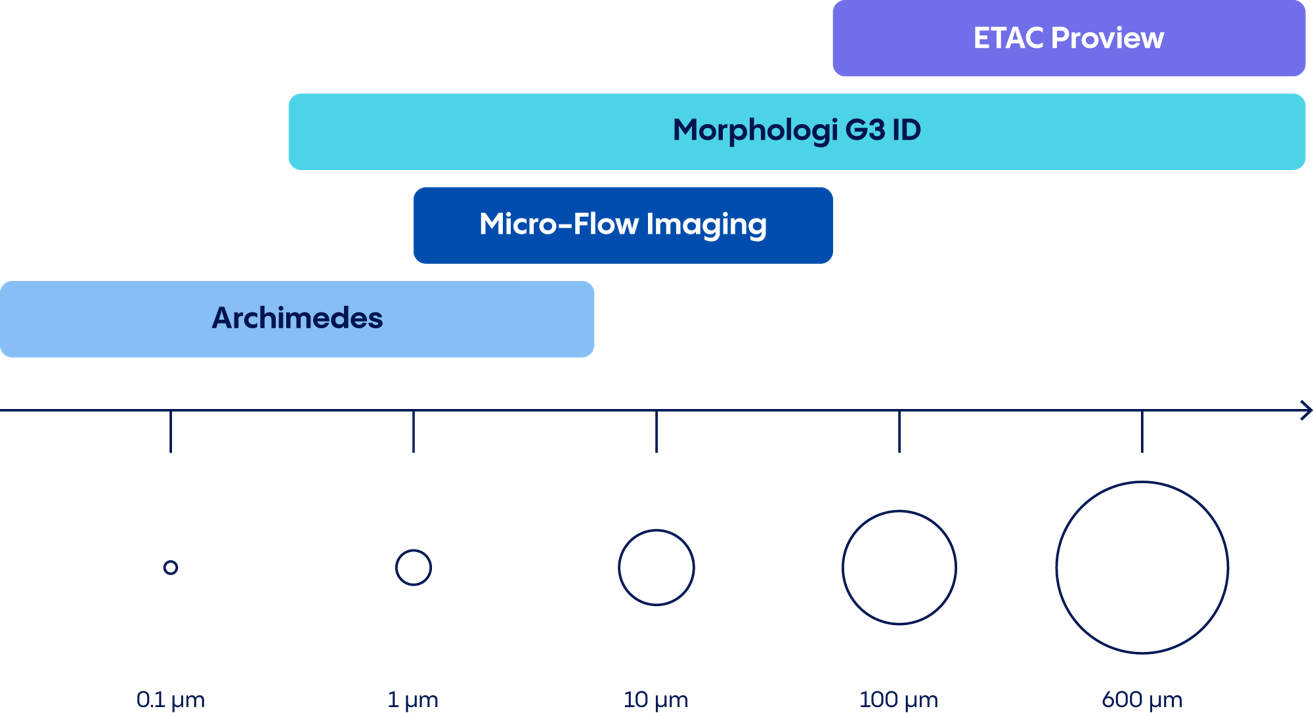
Micro-Flow Imaging:
MFI combines the possibilities of digital microscopy with modern microfluidics. This generates high-resolution images of sub-visible particles between 1 µm and 70 µm in size. Determining the number and size of the particles is then possible, as is particle characterization using modern application software via the morphology of the particles.
Resonant mass measurement:
Resonant mass measurement is a technique using the Archimedes system to detect sub-micrometer particles in a sample. A 50 nm – 5 µm particle flowing through a microfluidic channel changes the resonating frequency of a measurement needle, or cantilever, which allows the measurement of the buoyant mass, dry mass and size of the particle. Detecting particles with different buoyancy allows us, for example, to distinguish between protein aggregates and drops of silicone in a drug.
Digital microscope coupled with Raman spectroscopy:
The Morphologi G3-ID offers a unique technology: it combines the automated static imaging functions of a modern, high-resolution microscope, with the chemical identification of individual particles using Raman spectroscopy. This process can be used to classify a wide range of particle sizes (1 µm – 1000 µm) according to size and morphology, and to identify them chemically using the Raman spectrum.
Lab-scale digital visual inspections:
The ETAC Proview is a computer-based automated inspection system for use in research and development. It objectifies manual characterization by taking and saving high-resolution photos or movies of the filled units. With the help of innovative application software the visible mobile or immobile particles in each unit can be evaluated very precisely.
Vetter center of excellence for particle analysis
At the center of excellence for particle analysis of the Project & Service Analytics department, Vetter uses the most modern technologies and newest techniques. Our in-house expertise and comprehensive portfolio of services can help you meet the higher regulatory requirements in this particular field and continue maintaining the high standards in manufacturing quality.

