Vetter Success Story
It all started with a pharmacy in Ravensburg, 75 years ago. See the events, milestones and highlights that helped shape our history.
It all started with a pharmacy in Ravensburg, 75 years ago. See the events, milestones and highlights that helped shape our history.
We celebrate 75 years of Vetter and would like to take this special year as an opportunity to reflect on the most important milestones in our history and to celebrate the successes we have achieved together.
How can a vision turns into reality and an Upper Swabian pharmacy become a globally successful company like Vetter? When courage and passion meld, when pioneering spirit and expertise meet, and when those who get things done and those who make things possible come together. When the focus is on responsibility and the highest possible standard of quality. You can rely on Vetter—as you have since 1950.
In 1950, pharmacist Helmut Vetter leased the Marien-Apotheke pharmacy in Ravensburg and founded the Vetter Pharma family business. This decision laid the foundation for what Vetter is today. His vision and commitment to quality and innovation continue to guide the company and its employees today. We take pride in our heritage and are committed to improving the health and well-being of our patients around the world.








The state-of-the-art Vetter Training Center—which is connected to our Ravensburg West site—opened its doors in 2024. Its more than 5,000 square meters are allocated to training and development. 75 young adults complete apprenticeships to become a Chemical Laboratory Technician or Pharmaceutical Technician (m/f/d) over the course of three years. Here we also offer various certification programs and accredited IHK courses.
Vetter places a high value on learning and continuing education. For us, developing tomorrow’s professionals with the best resources we have to offer is integral to successful and sustainable growth.
Our scientific apprentices learn in a safe and immersive environment, which includes ultra-modern compounding simulation labs. This new facility also features nine modern classrooms, collaborative workspaces, and a dedicated VR learning space.








In 2023, few topics have occupied us as much as sustainability. While we steadfastly continue to grow, we have reached several important milestones in our sustainability strategy.
The prestigious recognition with the EcoVadis Platinum Medal was without a doubt one of the most important milestones on our mission to become a pioneer in ecological and social responsibility. And there were two more reasons for us to celebrate: We won the Sustainable Impact Award and received the Environmental Management Award from the German Federal Ministry for the Environment. In November 2023, we joined the Science Based Targets initiative (SBTi) with the aim of becoming 100 % carbon-neutral by 2050.
Find out more about our vision for a more sustainable future in the pharmaceutical industry in our sustainability report.








For years, we have been regulating and intensifying our extensive sustainability efforts with our EHS program (environment, health and safety). In 2021, we were able to achieve CO2 neutrality of all sites ‒ one of many achievements.
Our sustainability report, which was first published in 2022, is comprised of 82 pages and offers a detailed overview of all actions and a strategic perspective on Vetter's ecological, economic and social goals that should be reached by 2029.

After one and a half years of renovation, Vetter obtained the operating license for its new clinical production facility Vetter Development Service Rankweil in Austria's Vorarlberg region at the end of the year. The production site has been supporting international customers ever since during the important phase of process development and clinical filling of innovative APIs.

Despite an uncertain global outlook, we sustained our growth through a keen focus on balancing both stability and development. We continued our investment in both regional and international capacity (development and commercial), and opened a new corporate headquarters building in Germany.








Vetter reaches another important sustainability milestone: Thanks to relevant offsetting measures, our production emissions at all German sites have been CO2-neutral in Scopes 1 and 2 since mid-2019. These sites have been exclusively powered by CO2-neutral electricity from verified renewable energy sources.
This achievement highlights our commitment to protecting our climate and environment, and reflects an important element of our responsible corporate governance.

Our Center for Visual Inspection and Logistics wins the renowned Facility the the Year Award (FOYA), in the category “Facility of the Future.”
The International Society for Pharmaceutical Engineering (ISPE) is impressed by the intelligent combination of location design, state-of-the-art technology, and innovative processes.

With the establishment of aregional office in South Korea, Vetter underscores its commitment to the Asia Pacific market. The new office footprint better positions us to both support existing business in South Korea and help new local and global customers meet stringent development, manufacturing, and packaging requirements of their injectable drugs.

Expansion of our Ravensbug Schuetzenstrasse building helps us meet growing customer demand for drug development support, as well as our own need for enhanced IT systems.

A new Vetter business entity in Tokyo underscores Japan’s importance to our business. As the second largest pharmaceutical market, Japan is home to a number of leading global companies with promising injectable products in their pipelines. Our new regional office supports customer relations and the development of new business within Japan.

Vetter opens its first Asia-Pacific office to boost our regional presence and tap Asia’s rapidly growing healthcare market. The new office supports both customer relations and the development of new business.

This year marks the first of many clinical-to-commercial product transfers from the US to Germany, providing further proof of our versatile single-source manufacturing services.

Our new center for visual inspection and logistics goes into operation, with our Ravensburg West site offering even higher quality standards and safer, more efficient supply chain processes.
In many ways, the site’s optimized operations are an investment in the future: they use a variety of renewable power sources, including energy from both photovoltaic cells and biogas.

Vetter Development Service expands its operations and Vetter’s early clinical development services, with a site located at the Illinois Science Technology Park outside Chicago.
Now, Vetter can provide direct regional support to North American customers who need early-phase, cGMP-compliant aseptic filling of vials, syringes, and cartridges.

Our North American sales office relocates from Yardley, Pennsylvania, to a community outside Chicago, Illinois. Our subsidiary settles in the suburb of Skokie, future home of our first US production site: a new facility dedicated to early-phase clinical development.
Short commutes and a faster, more direct information exchange strengthens our partnership with regional customers.

On April 1, Vetter combines its sales and marketing activities under the subsidiary Vetter Pharma International GmbH, strengthening Vetter Pharma-Fertigung’s position in the international marketplace.
In the fall, Vetter opens its Ravensburg Vetter South site, including our new secondary packaging facility, VSP.

In 2008, the family business continues to work on its internationalization and professionalization and successfully transitions into the second generation. From now on, all Vetter families are represented in the company, and Senator h. c. Udo J. Vetter becomes chairman of the owner families.

Vetter becomes a full-service provider for its international customers. This new direction brings investment in new facilities, like Ravensburg Vetter South (RVS), as well as expansion of existing sites and implementation of new operational technology and quality assurance systems.

1990 is a significant year for Vetter. The company takes a major step forward by rolling out its fully automated production line and launching the Lyo-Ject® double-chamber prefilled syringe, an innovation that makes it possible to administer medication more safely and efficiently.
Two years later, Vetter uses robots in the aseptic production process for the first time ever, setting a new quality standard for parenteral product manufacturing. In 1996, production of the Vetter Lyo-Ject® dual chamber syringe is fully automated at our manufacturing facility in Langenargen.







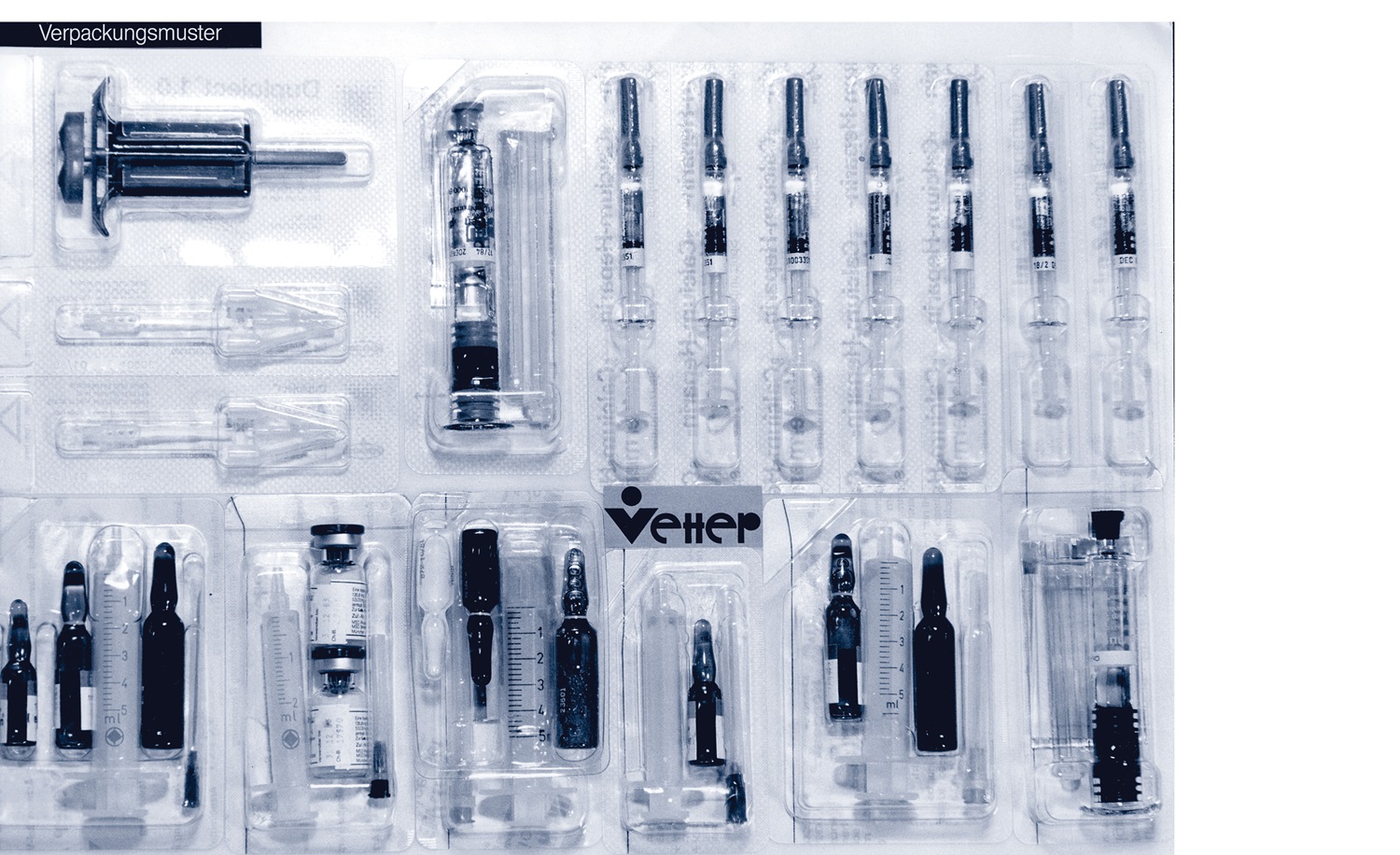
In 1981, Vetter takes the groundbreaking step of focusing exclusively on prefilled syringe technology. The company discontinues the production of contact lens fluids and halts the contract manufacturing of conventional dosage forms such as tablets, capsules, and powders
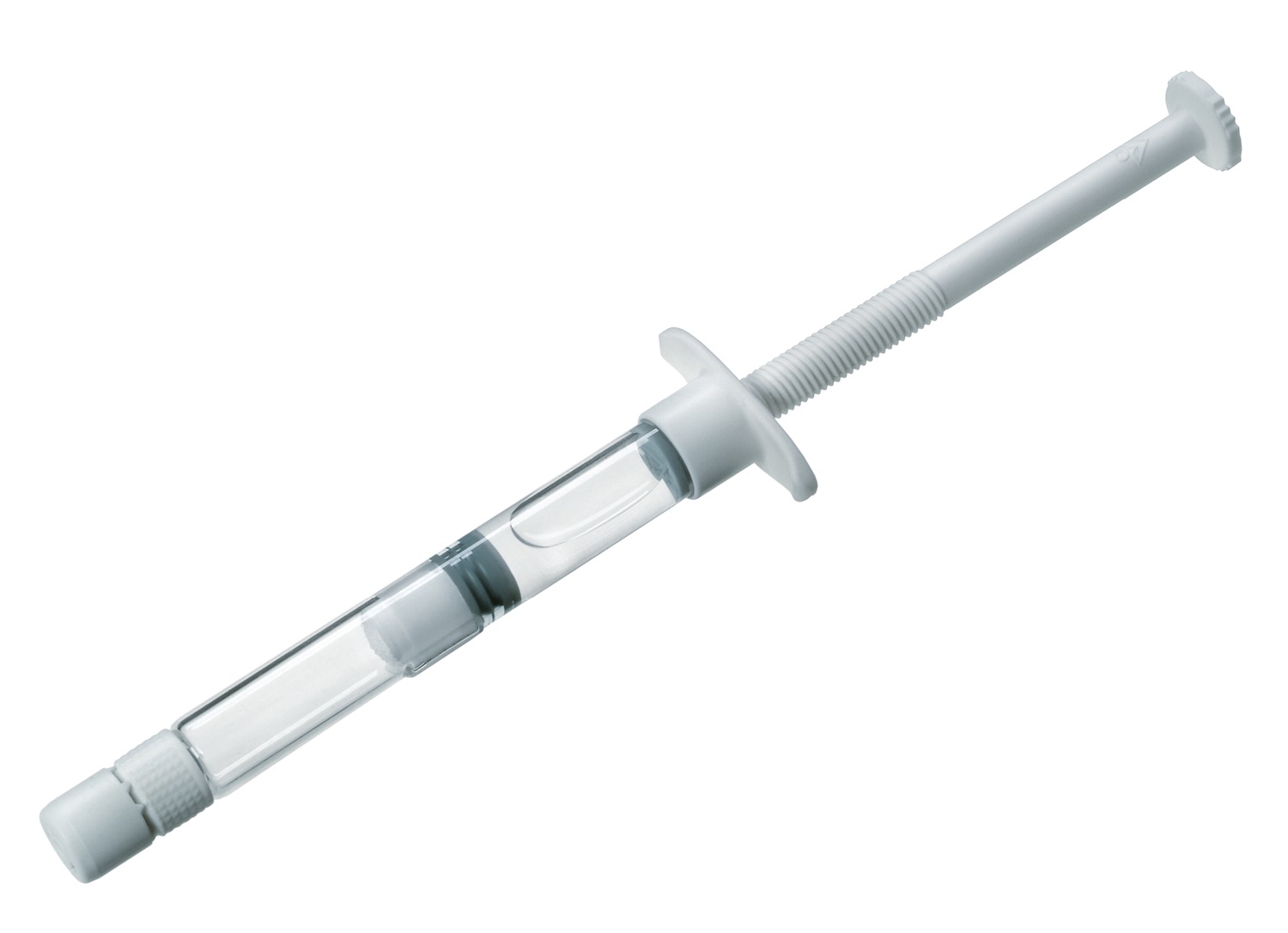
In 1979, Helmut Vetter has an innovative breakthrough: the pre-filled syringe, which quickly becomes the centerpiece of the company’s production strategy and forever alters how medications are administered.

In the 1960s, the quality of medicine packaging becomes increasingly important. In 1965, Vetter expands its services from packaging to include contract manufacturing of pharmaceuticals. In collaboration with a local engineer, the company develops innovative techniques.

This period marks an important milestone in the company's history. To keep up with demand, the company moves to larger premises, acquired the current Vetter pharmacy at Marienplatz, and begins producing additional medicinal products, special teas, and a teething gel.







In 1950, pharmacist Helmut Vetter leases the Marien-Apotheke pharmacy in Ravensburg and founds the Vetter Pharma family business. This decision laid the foundation for what Vetter is today. In 1954, next to the usual pharmacy business, he develops the stomach medicine Ullus® which is even exported to the United States. The advantage of its wafer capsules is that it is a tasteless form of gastrointestinal drug delivery.

Honorary Senator and pharmacist Helmut Vetter (1920-1999) founds the “Chemisch-Pharmazeutische Laboratorium Ravensburg GmbH,” which includes a laboratory, a school for lab technicians, and a drug production facility.
