Pharma and biotech companies undertake technology transfers for a variety of reasons. For most companies, it ensures an adequate supply for the patient. For others, it is to have in place a backup strategy for production or help overcome issues such as a lack of appropriate resources/capacity for process optimization, commercial production, secondary packaging, and/or supply chain management.
Tech transfer is also essential for scale-up projects that achieve better efficiency for manufacturing larger batch sizes.
Getting tech transfers right from the start
From a regulatory perspective, a tech transfer of an aseptic commercial product between cleanrooms may not differ much regarding time and effort compared to the initial development and design of the manufacturing process. However, it is determined by multivariable aspects, including filing strategies, special arrangements with authorities, and the commitment to accepting potential operational and methodical changes that enable the achievement of state-of-the-art processes.
Given this high degree of complexity, getting it right from the start is critical. The first step is the creation of a dedicated team that includes experts from development, production, quality assurance, regulatory affairs, quality control, and qualification/validation. The team is responsible for facilitating and executing the process and coordinating with the technology transfer/project leader.
Because there are multiple partners and sites involved in every tech transfer, numerous development steps and challenges are necessary. These include the sending unit - where a designated product, process, or method is expected to be transferred from - and the receiving unit - where a designated product, process, or method is expected to be transferred and executed.
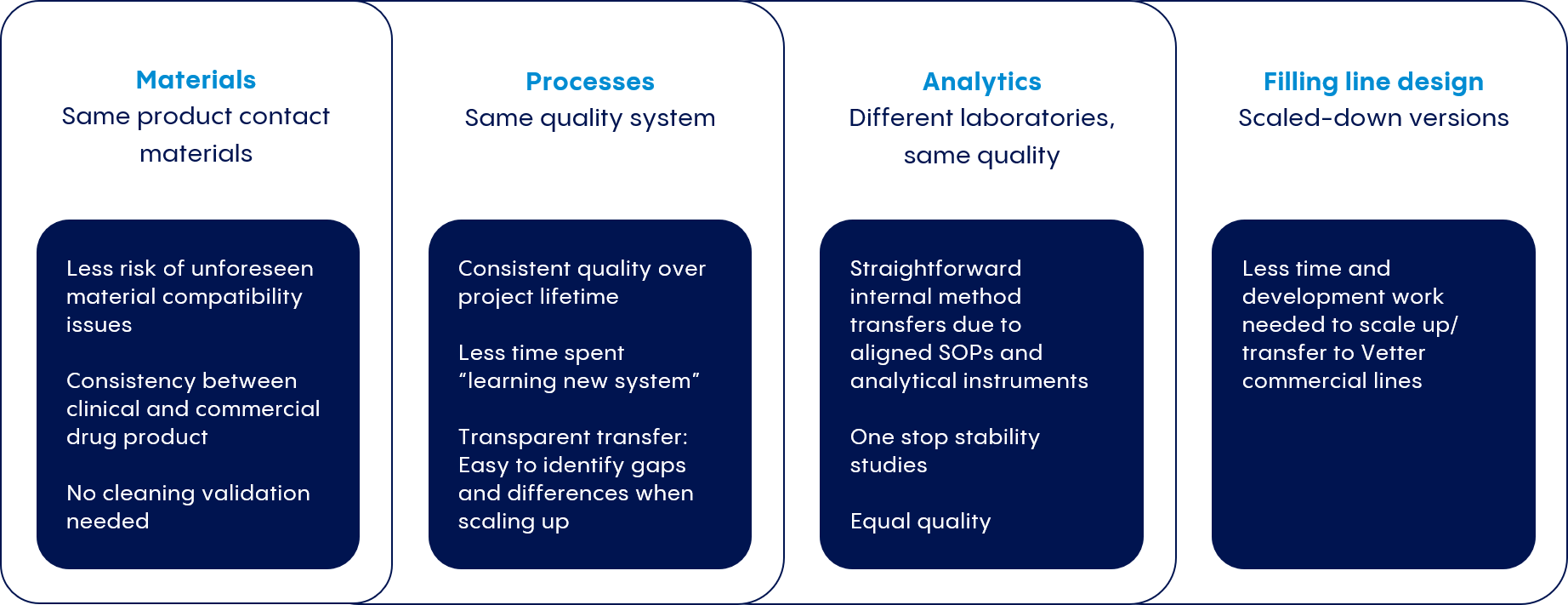
To protect timelines, cost projections, and regulatory compliance, we partner closely with our customers to develop processes that mirror those at our commercial production facilities.
Henrik Oberle, Vice President Development Service
Process transfers are based on the process validation approach of the US Food and Drug Administration’s (FDA’s) Guidance for Industry: Process Validation: General Principles and Practice. The steps involved ensure a robust and reproducible manufacturing process with consistent quality at the receiving site. These include:
Process development - initial comprehensive process development based on appropriate relevant documentation and the application of holistic risk management, according to International Council for Harmonisation (ICH) Q8 (R2) Pharmaceutical Development guideline.
Process design – knowledge gained through the development of the drug product and the experiences of scale-up activities that determine the manufacturing process to verify that the process delivers consistent quality for the product.
Process performance qualification - the evaluation of the process design to determine if it is capable of a reproducible commercial manufacturing approach.
Continued process verification – to ensure that the process remains in a state of control during routine commercial production for market supply.
The technology transfer is completed with the handover of the qualified process to the responsible production and quality units for commercial manufacturing.
Experience and Knowledge are Essential
In tech transfer, an intimate knowledge of the nature of the API as well as the quality attributes and formulation that result from events that may arise during stability testing is essential. This is especially the case since filing strategies in some countries have specific requirements for commercialization that can influence the scope of process qualification strategies.
Regulatory constraints can also influence the change in a production environment as can the exchange of processes e.g., equipment and facilities, cleaning and sterilizing procedures within or between production sites. The documentation of the transfer with all its contracts, protocols, reports, and instructions must also be completed. In-process and release specifications of the product must be adhered to and be comparable with the design space to achieve highest product quality.
Overcoming technology transfer challenges
As noted, there are many challenges involved in a successful technology transfer. The following example will help to illustrate but one difficult situation and how Vetter was able to successfully resolve it.
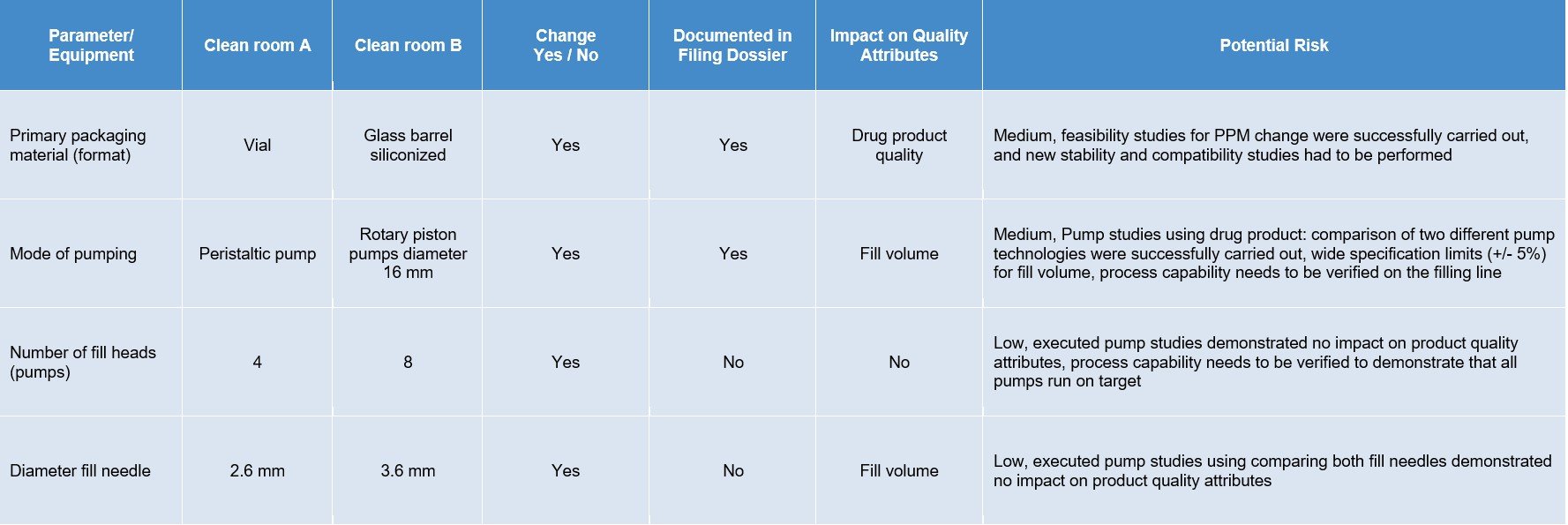
Based on a paper-based gap analysis, both the cleanroom processes were analyzed for differences in manufacturing steps and the equipment - e.g., format and preparation of primary packaging materials. One major change was foreseen for the primary packaging materials because the drug product went from a vial to a glass barrel. In this case, the washing and siliconization of the glass barrels, the siliconization level/distribution, and ejection forces were analyzed before entering the filling line.
The two main differences that had to be evaluated were siliconization distribution over the glass surface and content/functionality testing by determination of break loose and gliding forces interaction. Additionally, stability tests for a drug product solution with the silicon oil level—in contrast to a vial without silicon—were carried out. By using specific analytical methods, such as ultra-thin layer explorer and Fourier-transform infrared spectroscopy, the distribution was assessed to fulfill the requirements in combination with the measured break-loose and gliding forces to maintain the functionality of the product produced with the new cleanroom process.
Additionally, hold-up volume determination and a new design for expellable volume were carried out to fulfill the new requirements of the transition from vial to glass barrel. Because this change of primary packaging material had regulatory consequences, new stability and compatibility studies had to be submitted and adapted.
A skilled partner with the right expertise and people
Given its complexity and high risk, companies often rely on the expertise of a qualified CDMO with considerable know-how in process development and the technology to support a tech transfer/scale-up process to product launch. Tech transfer is only successful when the receiving unit can reproduce the transferred product, process, or method against a predefined set of specifications as agreed with the sending unit of the drug.
Thus, a project is ultimately dependent on the skill and performance of the individuals assigned to the project as well as the experience of the transferring team.
To ensure a smooth project execution, roles and responsibilities for all team members involved must be agreed upon, and a system that ensures adequate communication and feedback of information must be established.
Doris Rottenbusch, Team Manager Technology & Process Transfer
Vetter’s established transfer concept
We offer a holistic approach to transferring clinical manufacturing from our US-based facility in Chicago and Austrian-based facility in Rankweil to commercial fill and finish facilities in Ravensburg, Germany.