
Overcoming today’s aseptic fill-and-finish process challenges
Pharmaceutical service providers face an increasingly paradoxical challenge to their aseptic fill-and-finish process. Blockbuster drugs are in worldwide demand, as are the large-batch production runs they require. At the same time, intensifying research on rare diseases is also driving the demand for smaller batch sizes.
To meet the different requirements of both product groups, flexible manufacturing processes are of decisive importance—especially those processes that enable individual line set-up for product changeovers.
What’s more, many global authorities are also increasing their regulatory requirements for process safety. New and revised guidelines are upcoming. This includes the Annex 1 of the EU GMP Guide “Manufacture of Sterile Medicinal Products”—considered the most important European regulatory standard for the manufacturing of sterile pharmaceutical products.
Visual inspection requirements for particles are also becoming increasingly stringent. Regulatory trends are shifting from today’s best practice of “essentially free” toward the more demanding standard of “practically free.”
This have multiple consequences for the aseptic filling of drugs. First, it will increase demand for production technologies that can meet these stringent quality and safety requirements. Second, manufacturers need to anticipate future format, packaging and filling needs and, therefore will need technologies with sufficient built-in versatility.
Moving beyond conventional solutions
Today, two technologies are the foundation of high-quality aseptic drug processing: isolators and RABS.
Isolators are completely sealed units, entirely “isolated” from the outside environment. They are required to undergo extensive decontamination, which limits the level of adaptability and efficiency these facilities can achieve. RABS technology involves barrier and dynamic airflow separation between environment and drug product, while also offering the advantage of faster set-up, efficient product changeover, and variability. Consequently, RABS enables multi-product manufacturing operations with shorter downtimes.
With global demand increasing and regulatory requirements stiffening, drug manufacturers are responding with innovative new approaches that push beyond these two conventional solutions. Vetter Cleanroom Technology (V-CRT®) is one such approach. Vetter developed this holistic cleanroom concept combining the advantages of both isolators and RABS systems while also acknowledges all working steps related to aseptic filling—including decontamination, set-up and filling, monitoring, and analytics.
Designed to optimize the versatility and efficiency of pharmaceutical production processes, V-CRT® mitigates the risk of microbe carry-over from grade B to grade A, while still enabling rapid product changeover (see Figure 1).
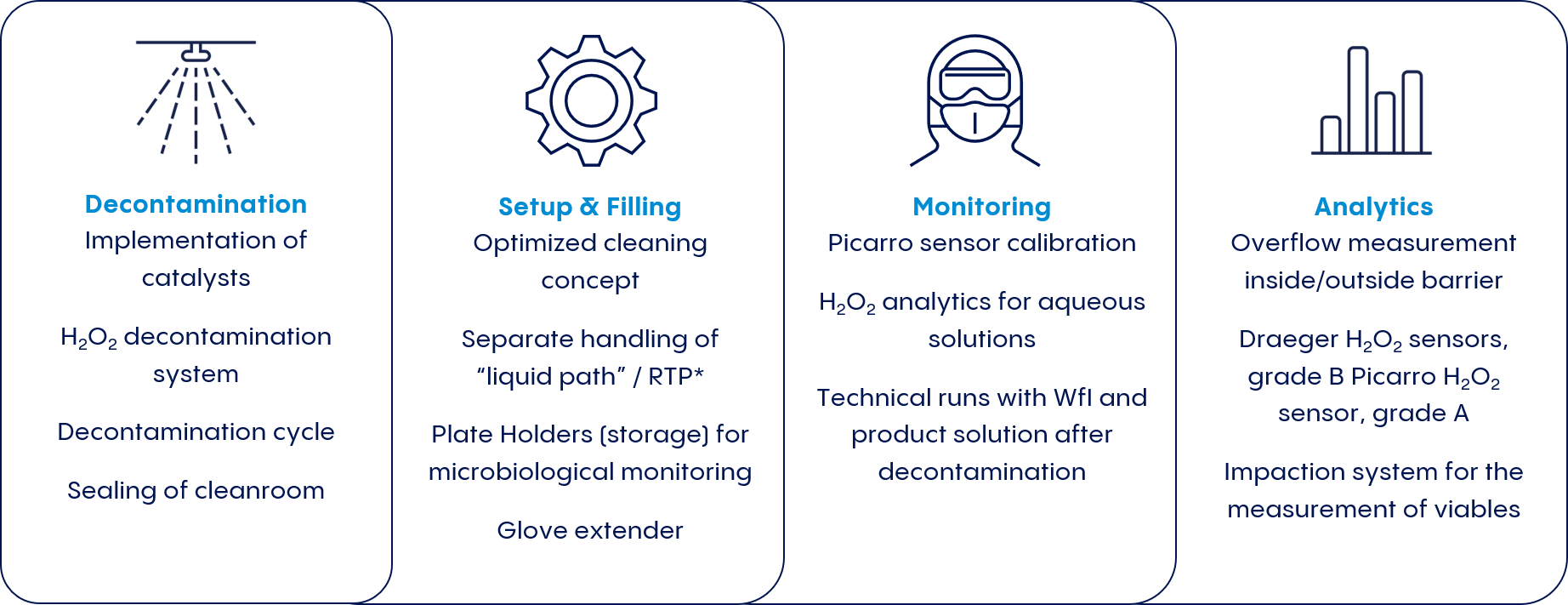
Rigorous preparation from the start
While automated H2O2 decontamination plays a crucial role, some important preliminary work must be considered first:
- Before automated H2O2 decontamination can begin, the cleanroom must be carefully prepared and machine surfaces must be wiped off.
- Equipment that do not touch the product and cannot be transported in a sterile box must be cleaned, sterilized, and installed in the cleanroom.
- Storage holders must be installed in the barrier and equipped with wrapped microbiological monitoring plates to avoid later transport from grade B to grade A.
- After closing the barrier doors, gloves and glove extenders must be installed and the remaining equipment parts must be brought into the barrier using sterile boxes.
Preparing the manufacturing line behind closed barriers in an aseptic environment further enhances the level of purity achieved through this system. All equipment parts brought in before or after decontamination are steam-sterilized in an autoclave before entering the cleanroom. The overall approach minimizes manual cleaning, reduces downtime, and increases overall equipment effectiveness (OEE).
In addition to these steps, further parameters are also monitored and controlled in preparation for the actual decontamination process (e.g. relative air humidity). Using inflatable door gaskets, the cleanroom is sealed air-tight. At this point, the actual H2O2 decontamination can begin.
The core of the concept: Automated H2O2 decontamination
Once decontamination begins, a system of stainless-steel pipes built into the cleanroom walls and ceiling aerosolizes the H2O2 solution into a class ISO 5 and 7 cleanroom through dual-substance jets inside and outside of the barrier. Thanks to these permanently installed nozzles, the entire cleanroom can be decontaminated automatically and autonomously.
After the required reaction time, the laminar airflow flushes the entire cleanroom. Appropriate catalysts ensure rapid decontamination, and a High Efficiency Particulate Air (HEPA) filter prevents particles from entering the room. The cleanroom is ready for use approximately 90 minutes after decontamination.
This automated approach eliminates the unwieldy processes and minimizes the sources of error associated with manual decontamination methods like formaldehyde fumigation. Compared with wipe-and-spray disinfection, automated H2O2 decontamination also provides greater protection against microbiological contamination. H2O2 eliminates critical grade A microorganisms and is practically residue-free, since the solution quickly breaks down into water and oxygen (see Figure 2).
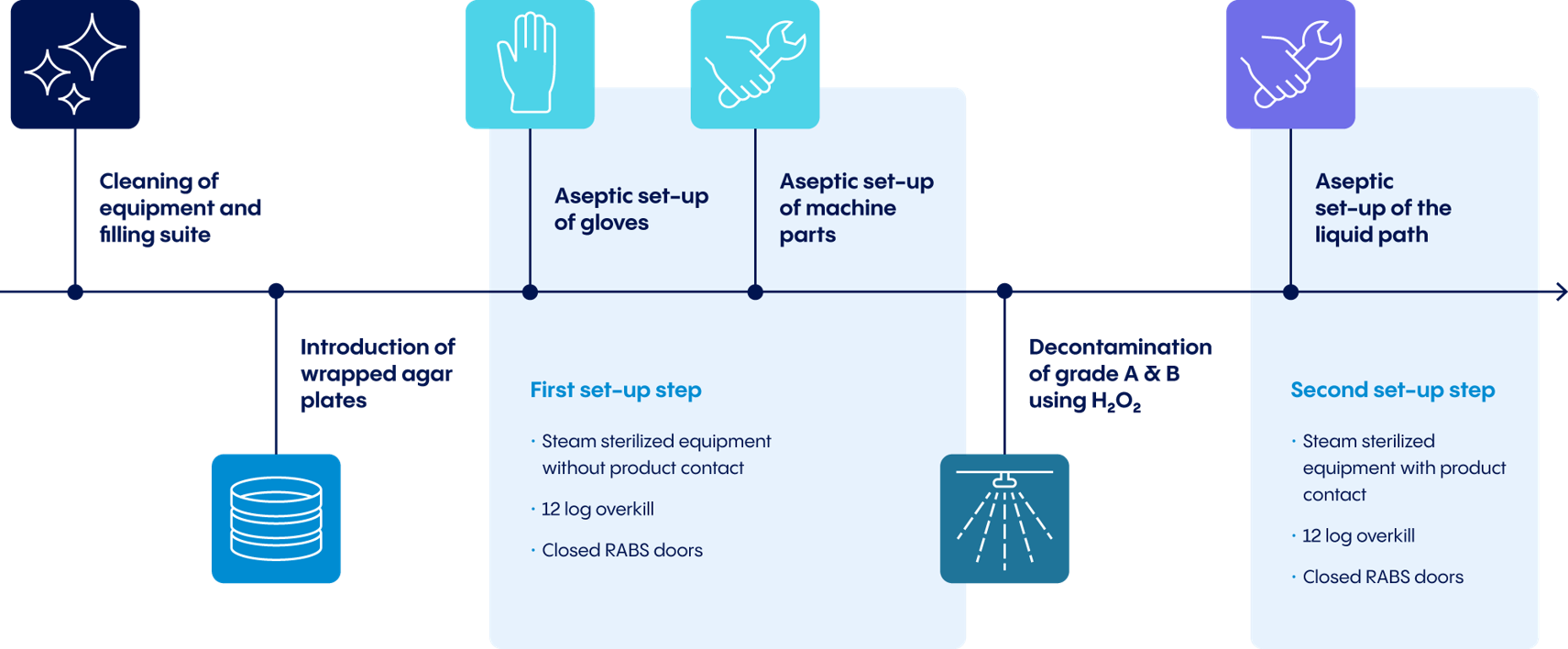
Continuous monitoring and analytics
A holistic cleanroom concept integrates monitoring and analytic measures that not only track the aseptic filling process, but also ensure that both products and employees are fully protected from H2O2. Sensors inside and outside the barrier are tuned to track different variables.
Two Polytron sensors measure H2O2 decay within the cleanroom in grade B, while a Picarro sensor follows the same process in grade A. A flowmeter continuously measures the air flow from inside the barrier area into the ambient cleanroom air.
The analytics concept also includes H2O2 analysis for aqueous solutions. Thus, H2O2 concentrations in WfI (water for injection) or other product solutions can be verified in the lab.
Prepared for tomorrow’s challenges
In addition to high reliability and reproducibility, this cleanroom concept also offers the significant advantage of speed. Depending on the size of the cleanroom, the entire decontamination cycle can be completed in just 2.5 hours.
Suitable catalysts and a highly effective ventilation system enhance H2O2 degradation and lead to short decontamination cycles. Preparations for production and the aseptic set-up of the cleanroom can begin immediately. Shorter downtime streamlines processes and improves overall equipment effectiveness (OEE).
In the near future, pharmaceutical product manufacturing will require significantly increased flexibility and face more stringent requirements for microbial and particulate contamination control. Pharmaceutical and biotech companies around the world are already feeling these pressures on their aseptic filling processes. But many of these organizations are already adapting their strategy accordingly: They’re taking advantage of innovative production methods, designed to enhance and advance current manufacturing processes.
Leveraging these new methods can help pharmaceutical and biotech companies prepare for success in a changing and challenging industry. New approaches like Vetter’s V-CRT create manufacturing processes that deliver all benchmarks—safety, versatility, efficiency, and above all, quality—to meet the ever-evolving demands of the world’s dynamic healthcare market.
About the author
Ute Schleyer, Ph.D. was appointed Project Manager Site & Plant Development of Vetter Pharma-Fertigung GmbH & Co. KG in 2016 and is responsible for supporting projects in the field of pharmaceutical technology. Ute joined Vetter in 2007 as Manager of Aspetic Production. She was promoted to the position of Head of Production in 2008 in the area of manufacturing and filling of sterile drug products, which includes various single and dual-chamber filling lines. Ute holds a Ph.D. in biology from the Johann Wolfgang von Goethe-University Frankfurt, Germany.
First published on 10/14/2020 in Contract Pharma
Related Videos




