
Bringing a new drug product to market can be a major challenge when it comes to guiding the late-stage development through to commercial readiness and launch. In fact, this is often considered one of the most impactful milestones of any drug program, because it culminates years of development work.
It usually is during late-stage development where there is a mindset shift from developing a drug product, to commercializing it. Since timing is a crucial factor, decisive and focused launch management is required and involves a dedicated and experienced team to coordinate all necessary process steps. A successful first commercial production can be achieved in a predetermined timeline by following a thorough step-by-step approach.
The biopharma product launch landscape is very diverse, and as such, the needs of each product to reach the market can vary significantly. To effectively establish a plan for launch management, the Contract Development and Manufacturing Organization (CDMO) partner need to work closely with the customer to decide on the product-specific process.
Part of the launch is designing a tried-and-true methodology to meet the specific needs of each molecule to bring both, a small batch as well as a blockbuster drug to market. Throughout the launch management process, the team is working against the launch date with the intentions to follow the best product implementation strategy for commercial market supply.
Generally, when customers prepare for launch, they have already been collaborating with a CDMO partner and have an assigned project manager who helps guide their drug product through clinical development. When the launch management process begins, customers are paired with a launch manager, in addition to their project manager, to guide them through the journey to commercial production.
At this point, the project manager assumes a new role within the launch team, and the launch manager takes the lead. The customer has access to both, the project and launch teams throughout the journey to commercial readiness. Though the managers will support the seamless step-by-step approach, external factors such as material or API supply must be closely monitored to avoid challenges that may arise and are outside the control of the service provider and customer.
Transparency and open communication on risks and active management of expectations are paramount to make sure all parties involved agree on each process steps. A proven step-by-step approach contributes significant value to achieving market launch on time and allows the customer and partner to keep track of several activities running in parallel, preventing overlooked gaps or missed milestones.
Initiation – Define Launch Process and Timeline
The launch management process is initiated based on the customers product launch plan. This phase allows both parties to define the organizational structure as well as the requirements and expectations for the timeline to bring the product to market. With large teams from various departments such as quality control, demand management and supply chain management involved in the process, it is important to identify the roles and responsibilities of each member. This applies to the filling partner as well as the customer launch teams.
Launch Kickoff – Initiate Product Handover to Commercial Team
The kickoff follows the start of product transfer from project to commercial. This phase includes internal and external kickoff meetings to get all relevant stakeholders involved in the process. During the latter, the service provider introduces the launch management process and provides a full project and product overview to the launch teams. The customer in turn provides a full overview on their final product set up for commercial and their understanding of launch management.
This is also when the project manager, launch manager and customer will align on milestones, common goals, timelines and identify risk factors. Once the project manager hands the lead over to the launch manager, they confirm a joint understanding of the overall approach, the future commercial working frame, and the supply chain management process. Assuming there is agreement on the communication and core team setup, the kickoff is complete.

Launch Phase – Establish Commercial Set-Up
During the launch phase, a filling partner will establish a methodical way of working with the team including both internal and external players. This step also includes gaps and opening actions within a launch checklist developed in collaboration with the customer. Progress made throughout the launch phase is tracked during the regular meetings both internally, within the CDMO, and externally with the customer, to maintain open lines of communication.
It is common during this phase to evaluate both best-case and worst-case forecasts to correspond with the planning of the launch and to develop corresponding scenarios to support flexible handling of the process throughout.
In an effort to conduct commercial setup, the launch management team will allocate process topics e.g. the API allocation, setup primary packaging, management supply, API storage and cleanroom equipment to match the customer’s forecasting needs and logistics process. Throughout commercial setup, there is an overall awareness of the anticipated timeline and processes, as well as dependencies on both the CDMO and the customer.
As the process goes on and milestones are met, action items on the launch checklist are checked off. If there are any risks, whether related to supply chain, packaging, or otherwise, that might delay launch, they are flagged in this phase of the process.
Launch Batches – Manufacturing First Commercial Batch
This phase marks the completion of product transfer from project to commercial teams and the execution of the first commercial fill within the pre-planned scenario. Most of the defined activities must be completed by the launch tracker such as packaging material availability, personnel training and adherence of correct shipping processes and requirements. Also, all necessary orders are placed, and the availability of crucial components are confirmed.
Post-Launch – Commercial Manufacturing and Project Close Down
Following the successful release of the first launch batch, a formal close-down of the project commences. The CDMO team will collect lessons-learned and feedback from the customer to optimize execution of future launches. Learning occurs on both sides - the customer learns how to better get their products to launch, and the filling partner learns how to further improve its launch management process.
Once a launch batch of the drug product is released, the formal close-down within the system is complete and the project manager and project team are released. At this point, the product has made its way through the launch management process and is now in the hands of the commercial team.
The commercial team will remain responsible for continuous process verification, monitoring key performance indicators and yield data, forecasting and capacity reservation for medication, participating in business review and quality management review meetings, and managing the future lifecycle activities of the product.
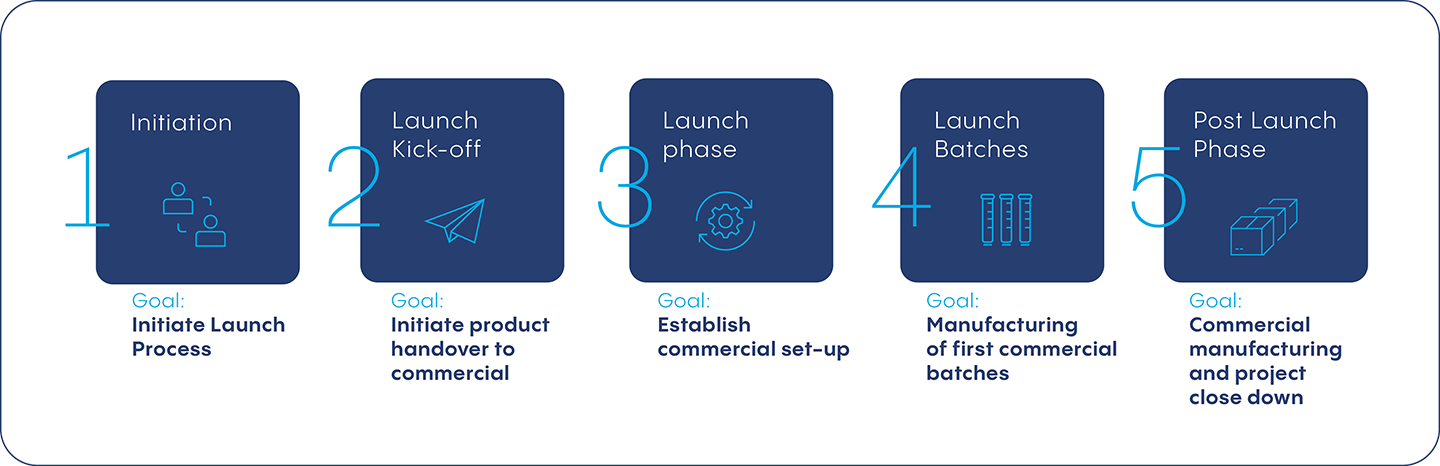
Vetter, one of the world’s leading fill and finish CDMOs for injectables, established this 5-step process for maximum efficiency when bringing a drug product to commercialization. This process offers a tried-and-true methodology for getting a small batch as well as blockbuster drug product to market.
Nevertheless, this process requires continued adaptation and adjustment to align with environmental factors. As a result of these external obstacles, Vetter bases success on a plannable and structured process, that provides reliability for customers on the launch management timeline set at the start of the journey.
This reliable step-by-step process has supported customers’ success, big and small, for both startup biotech companies and pharma giants, and will be further adapted to continue doing so in the future.
The article was first published on contractpharma.com
About the authors
Dr. Anna Schamberger, Director Customer Project Management
Dr. Schamberger has 10+ years of experience in the pharmaceutical industry, primarily working with CDMOs. After joining Vetter in 2016, she began in customer Project Management leading late-stage DP and life cycle projects. Since 2020, Dr. Schamberger has been a director in customer project management leading a team of eight project managers. She is also a program manager for strategic partnerships overlooking and leading product portfolio and supports multiple key strategic and operational activities within Vetter’s customer project management, achieving customer goals to commercialize innovative and life-saving products for a global market.
Sabrina Schumacher, Team Leader Customer Service
Ms. Schumacher has 15 years of experience at Vetter and over seven years of experience as a Customer Relations Manager, leading several launches for various customers in the function of a Launch Manager. She supports customers as a central contact and knowledge carrier within the company and develops solutions to complex challenges throughout the commercial development phase. Since 2020, she has been the Team Lead Customer Service leading a team of nine customer service representatives.


