
One of a drug developer’s greatest responsibilities is to ensure that the products they provide are safe for use in humans. No matter how a medicinal product is delivered – through injection or any other way – it must be manufactured in a way that meets rigorous quality and regulatory standards.
Two of the most common standards that guide how drug products are manufactured are Good Manufacturing Practices (GMP) and Current Good Manufacturing Practices (cGMP). Here are some important differences between these two standards, and what those differences can mean for manufacturers.
GMP: A foundation of quality for drug products
Established by the US Food and Drug Administration (FDA), GMP is a set of core best practices for manufacturing products intended for human use. Drug owners around the world use GMP as the foundational guidelines for producing high-quality medications, both to protect their products’ quality and to help ensure regulatory compliance.
GMP protocols broadly define appropriate standards for many key processes, resources, and controls involved in the drug manufacturing process. Several examples include:
- Facilities, equipment, and technology
- Cleaning and sanitation
- Documentation and recordkeeping
- And much more
One important thing to know about GMP is that it is not a group of strict rules or exact specifications.
Rather, it’s a set of general, open-ended principles that give manufacturers the flexibility to determine the most efficient and effective methods to meet the indicated standards in a compliant way.
cGMP: Promoting continual advancement

While GMP guidelines help set core, baseline quality standards for drug products, manufacturing science, technology, and regulations are constantly evolving. Methods and equipment that were considered “state-of-the-art” 10 years ago may still support GMP compliance, but may not enable the most effective or efficient processes today.
Since 1978, the FDA has referred to the GMP guidelines as cGMP to emphasize that the regulations are constantly evolving to reflect the latest developments in the industry.
The "c" stands for "current": manufacturers who comply with cGMP must not only meet GMP standards, but also do so using the most modern and up-to-date methods. Therefore, new guidelines are constantly being published as part of the cGMP compliance.
These new guidelines are not part of the law like the basic GMP. However, they are considered as current GMP and are part of any authority inspections.
These guidelines are more detailed, rigorous and demanding, and cover many more specific components in the drug manufacturing process. Some important examples include:
- Data Integrity
- Aseptic Processing
- Contract Manufacturing
- Risk Management
- And much more
Like GMP, cGMP guidelines provide flexibility in meeting standards. However, cGMP-compliant drug manufacturers must not only meet quality benchmarks but also use the latest methods available during manufacturing.
This requirement promotes continuous improvement and ensures that manufacturers do not rely on outdated methods.
GMP vs cGMP: Key differences to consider
Here are some key differences to consider deciding whether to pursue GMP or cGMP compliance:
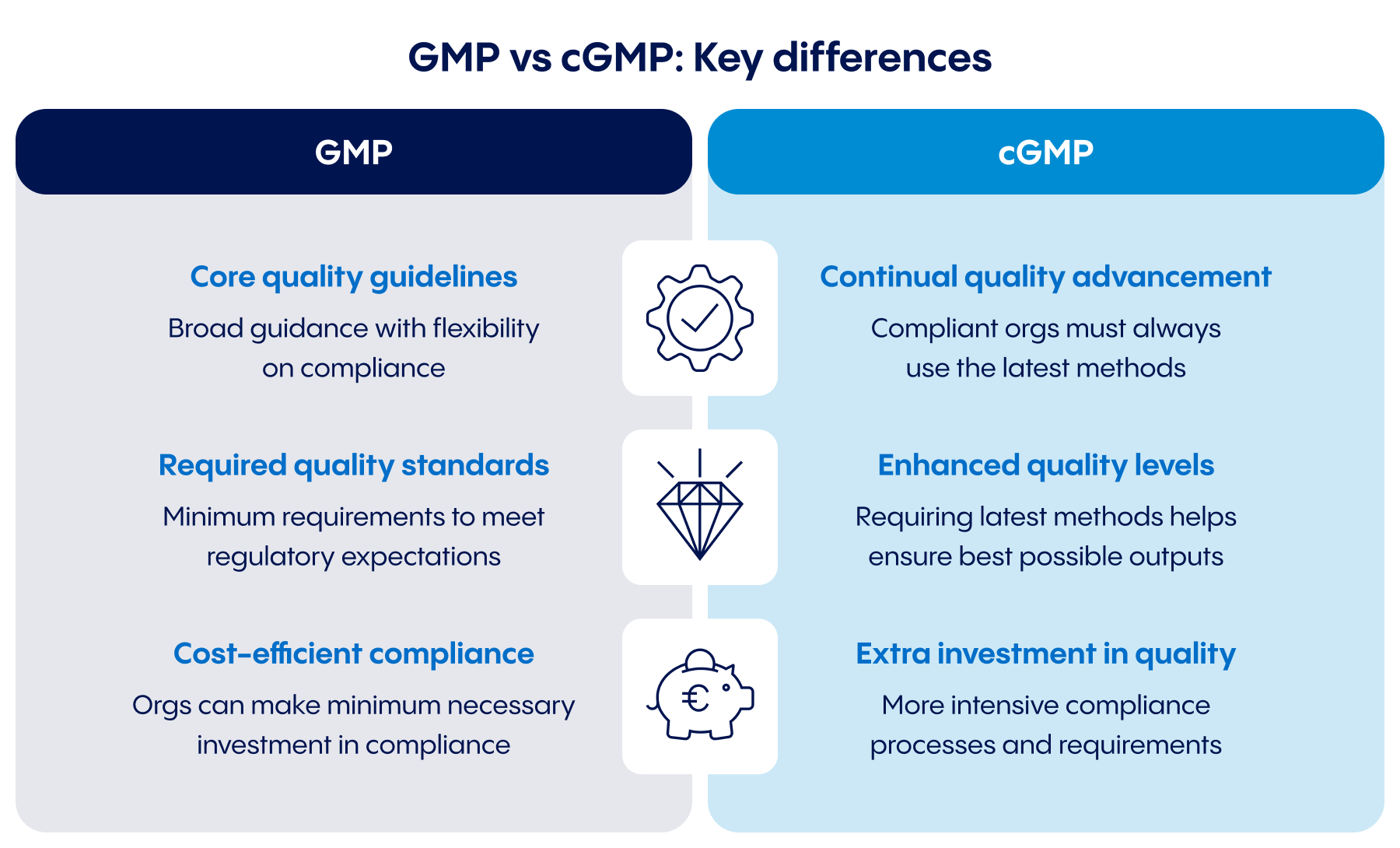
Core quality guidelines vs continual quality advancement
GMP provides broad guidelines that are regularly updated, but offers manufacturers flexibility in how they meet those standards. cGMP demands continual evolution: compliance requires that a product be manufactured using the most current procedures possible. Pursuing that more demanding standard requires greater investment in technology, training, process evolution, and more.
Required quality standards vs. enhanced quality levels
Complying with GMP can help drug owners and manufacturers meet minimum quality standards for many different markets and regulatory frameworks. Today, however, regulators expect more. Complying with more rigorous cGMP regulations can help pharmaceutical companies achieve higher-quality outputs with fewer deviations – and prepare to pass higher levels of scrutiny.
Cost-efficient compliance vs. extra investment in quality
GMP gives drug owners and manufacturers the leeway to produce a medication in the most efficient way possible. In comparison, cGMP compliance is typically more expensive due to the advanced infrastructure, innovative technology, and demanding processes these additional guidelines require, as well as the investment in continual improvement.
Where to start with cGMP compliance

Because of these added benefits, striving for cGMP compliance can be a smart long-term strategy for a company developing a new injectable drug product. Despite the higher costs, complying with cGMP can signal a commitment to quality, minimize regulatory friction, and show potential licensing partners that a product is manufactured to the highest possible standards.
At the same time, it’s important to be aware that this approach requires a much more demanding compliance program.
To successfully implement and maintain cGMP processes, companies must continually monitor the scientific, regulatory, and technological landscape for changes in current best practice, and must make any modifications or updates per required implementation date of the new guideline.
Regulators who discover outdated methods or techniques may pause production of the product until compliance is restored.
To support effective, ongoing cGMP compliance, it’s important for these guidelines to be integrated into the appropriate processes and controls as early as possible. Doing so will help create a strong, strategic quality framework around the product - one that can support regulatory submissions, streamlined life cycle transitions, and many other successes.
Key components of GMP and cGMP compliance
Once the decision has been made to pursue GMP or cGMP compliance, three core components of this standard are typically the best ones to focus on first: qualification, validation, and documentation.
- Qualification is the process of matching a drug product with an optimal combination of manufacturing technology, processes, and trained experts.
- Validation focuses on verifying the consistency and repeatability of the manufacturing process.
- Documentation includes putting processes in place to ensure that every step of the manufacturing cycle is meticulously tracked and captured in an accessible form.
To learn more about the role each of these steps plays in a cGMP-compliant manufacturing process, watch this special feature on the Vetter Video Hub.

Looking for more guidance on GMP and cGMP?
While Qualification, Validation, and Documentation are vital to cGMP compliance, developing a fully compliant program requires an expert partner who has extensive experience with implementing cGMP guidelines for sterile injectable products.
If you’re ready to pursue the highest possible quality standards for your injectable drug product, reach out to Vetter's experts.

About the author
Dr. Gerhard Reuter is a cGMP specialist who plays a critical role in authorizing the release of both clinical and commercial batches. He has decades of experience with establishing and maintaining quality systems for drug manufacturing processes.


