Production Excellence
Pursuing the highest possible standards
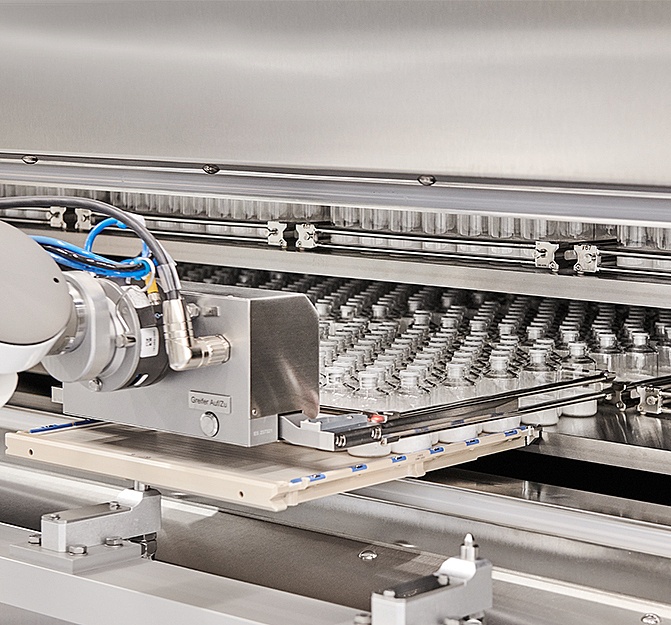
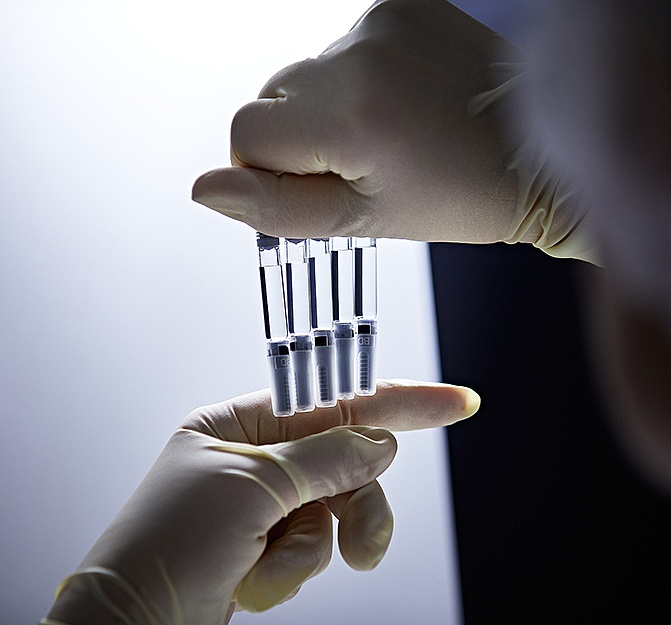
When it comes to our customers’ commercialized products, we bring our best to every process and output. Today, that passion for performance is more important than ever – as regulatory burdens grow and market forces drive demand for efficiency and flexibility.
That’s why we’ve established our Production Excellence program: our internal roadmap for ongoing optimization, alignment, and development of our fill and finish processes. From compounding, to material preparation, filling, environmental monitoring, and beyond, this framework keeps our services on a path of continual evolution.